23+ How To Determine Rate Law
Solved Examples on the Rate Law Example 1 For the reaction given by 2NO O2 2NO2 The rate equation is. The reaction order is the sum of the concentration.

Determining Rate Equation Rate Law Constant Reaction Order From Experimental Data Video Lesson Transcript Study Com
On a 30-year jumbo.
. The equation for a component A is rate kAm where m is the. The rate law for a chemical reaction can be determined using the method of initial rates which involves measuring the initial reaction rate at several different initial reactant concentrations. Compose the rate law expression with just the reactants.
The equation can then be. Determine the reaction order with respect to the first reactant. Given the reactant orders.
Determine the values of m n and k from the experimental data using the following three-part process. Rate kNO 22CO0 kNO 22 Remember that a number raised to the zero power is equal to 1 thus CO 0 1 which is why we can simply drop the. The rate law for a chemical reaction can be determined experimentally by measuring the reaction rate at different concentrations of reactants.
Determine reaction order with respect to the. The court is being asked to determine the constitutionality of Texas and Florida laws letting users sue online platforms for alleged political censorship CNN values your. For a reaction such as aA products the rate law generally has the form rate kAⁿ where k is a.
One way is to use the method of initial rates. Steps for Writing a Rate Law Expression Given Reaction Order. Draw a graph of the data.
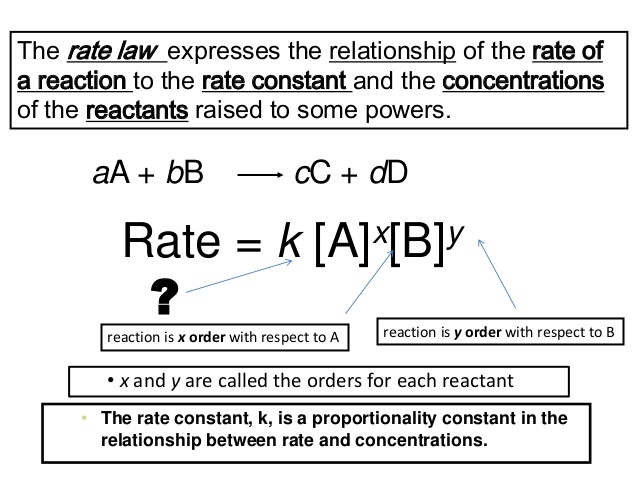
A rate law shows how the rate of a chemical reaction depends on reactant concentration. The rate law will have the form. The rate law expression is not determined by the balanced chemical equation.
2 hours agoThe 2023 Canadian Law Firm Hourly Rate Report is the most comprehensive and detailed competitive intelligence and legal pricing tool available because the report details. The rate law can be determined from a table of experimental data by using the following steps. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations.
Determine the value of m from the data in which. Determining Rate Laws from Experimental Data TheChemistrySolution 584K subscribers Subscribe 29K views 2 years ago This tutorial covers how to determine the overall rate law for. The rate law is.
Steps to Determine a Rate Law Using Initial Rates Data Step 1. If Rate is given by k A x B y the overall order of the reaction n x y. A rate law shows how a change in concentration affects the rate.
Find the best-fit line for the data. Rate k NO2O2 Find the overall order of the reaction and the units of the. On a 30-year jumbo.

14 3 Concentrations And The Rate Law Youtube

Solved Can You Help Me Get The Correct Answers And A Brief Chegg Com
5 2 Methods Of Determining Reaction Order Chemistry Libretexts
The Simulated A And Measured B 2d Avoided Crossing Spectrum Of The Download Scientific Diagram
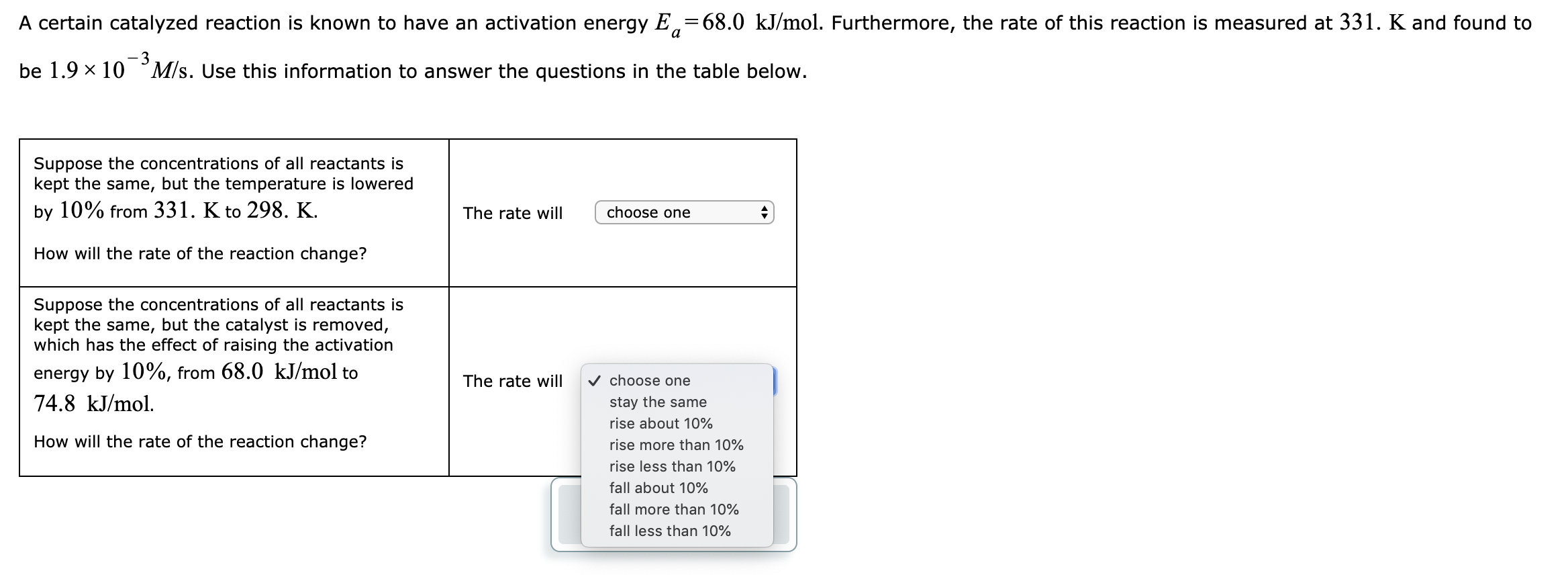
Answered A Certain Catalyzed Reaction Is Known Bartleby
Schematic Of The Experimental Setup For Ion Ion Coupling Via A Common Download Scientific Diagram

Rate Law And Reaction Order Video Khan Academy

The Rate Constant Of A First Order Reaction Whose Half Life Is 480 Seconds Is

From Metal Binding To Nanoparticle Formation Monitoring Biomimetic Iron Oxide Synthesis Within Protein Cages Using Mass Spectrometry Kang 2009 Angewandte Chemie International Edition Wiley Online Library
Factors Of 23 Prime Factorization Methods Tree And Examples

Determining Rate Equation Rate Law Constant Reaction Order From Experimental Data Video Lesson Transcript Study Com

Determining Rate Equation Rate Law Constant Reaction Order From Experimental Data Video Lesson Transcript Study Com

Homogeneously Catalyzed Electroreduction Of Carbon Dioxide Methods Mechanisms And Catalysts Chemical Reviews
Rate Constant Calculator Online Solver With Free Steps

Rate Constant And Rate Laws Video Lesson Transcript Study Com
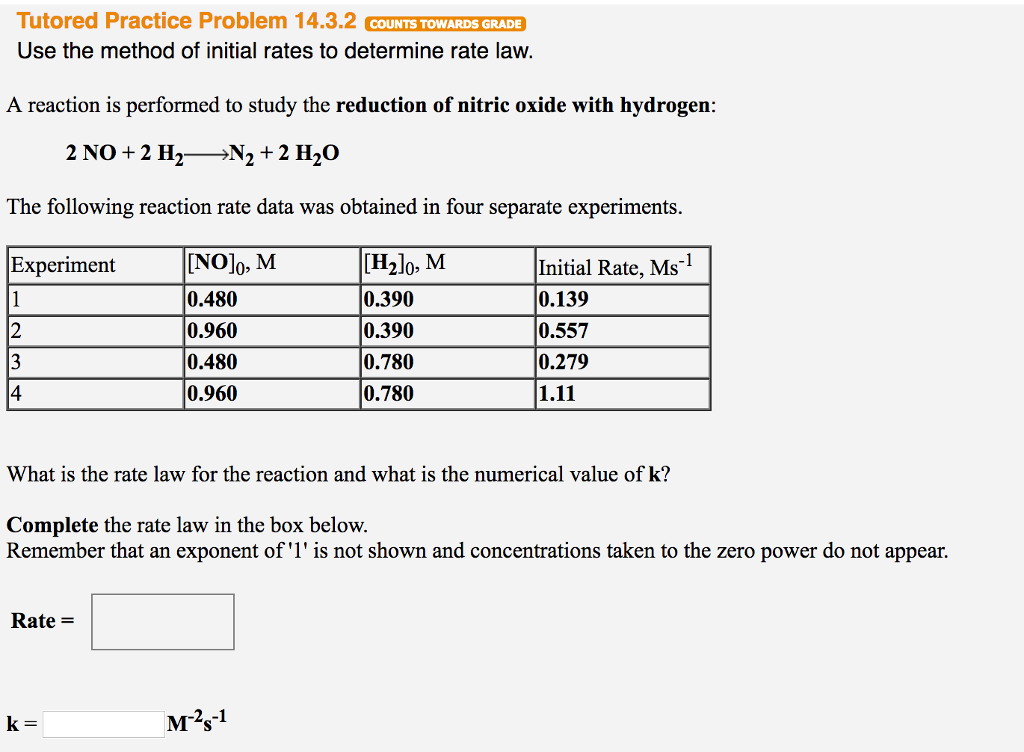
Solved Use The Method Of Initial Rates To Determine Rate Chegg Com

Kinetics How To Determine The Rate Law Knowing Time And Concentrations Chemistry Stack Exchange